Introduction: Understanding Gut Disruption and the Role of Probiotics
In the intricate ecosystem of the human body, the gut plays an extraordinary role in maintaining both digestive and immune health. Yet one of the most common disruptions to this complex system arises from a necessary yet often indiscriminate source—antibiotics. While these medications are essential for combating bacterial infections, they also wipe out beneficial microorganisms that help sustain the gut microbiome. This dual impact has led many to seek strategies to counterbalance the damage, and one of the most effective and scientifically supported approaches is incorporating the best probiotic for antibiotics into one’s health regimen. These probiotics are more than supplemental additions; they are targeted tools for recovery, resilience, and long-term digestive harmony.
Antibiotics have revolutionized modern medicine, saving lives by eliminating dangerous pathogens. However, they are not selective in their microbial purge. This can result in a weakened gut lining, reduced microbial diversity, and an increased risk of opportunistic infections such as Clostridioides difficile. For many, this manifests as gastrointestinal discomfort, including bloating, diarrhea, and reduced nutrient absorption. These outcomes have led researchers and clinicians to explore how probiotics—live microorganisms that confer health benefits when administered in adequate amounts—can assist in restoring balance. Using probiotics with antibiotics has evolved from a fringe concept to a medically endorsed practice backed by emerging data and real-world application. Through a closer examination of how these microbial allies work, what specific strains are most beneficial, and how timing and delivery affect efficacy, we can better understand how to navigate antibiotic recovery with informed, evidence-based choices.
You may also like: The Ultimate Guide to Choosing an Effective Immune Support Supplement for Daily Wellness
Why the Gut Microbiome Matters in Antibiotic Recovery
The gut microbiome consists of trillions of bacteria, viruses, fungi, and other microbes that live symbiotically within our intestines. These microbial communities perform crucial functions: they aid in digestion, synthesize essential vitamins, protect against pathogenic invaders, and play a key role in immune system modulation. When antibiotics are introduced into this environment, they often eliminate both the harmful bacteria and the commensal, beneficial strains that keep the gut balanced. This disruption can impair mucosal immunity, weaken gut barrier integrity, and alter metabolic pathways critical to nutrient assimilation and neurotransmitter production.
What makes this disruption especially concerning is that the gut microbiome isn’t just responsible for digestive health. Studies have shown that the composition of gut bacteria influences mental health, autoimmune conditions, metabolic diseases, and even cardiovascular function. Therefore, a compromised microbiome can have ripple effects throughout the entire body. The goal during and after antibiotic treatment should be to restore microbial diversity as efficiently and thoroughly as possible. This is where probiotics for taking antibiotics become indispensable. Not only do they help reseed the gut with beneficial microbes, but they also help crowd out opportunistic pathogens and reduce inflammation. They are not a mere supplement—they act as a biologically active intervention during a period of vulnerability.
Using Probiotics with Antibiotics: How It Works and Why It Matters
The concept of using probiotics with antibiotics may initially seem counterintuitive. After all, if antibiotics are designed to kill bacteria, wouldn’t they also destroy any probiotics taken concurrently? The answer lies in timing, strain selection, and formulation. Clinical studies suggest that probiotics can be taken safely alongside antibiotics if properly timed—typically several hours apart—to avoid direct antimicrobial conflict. More importantly, specific strains have been identified that demonstrate resilience in the face of antibiotic exposure.
For instance, Lactobacillus rhamnosus GG and Saccharomyces boulardii are two of the most extensively researched strains with proven efficacy in preventing antibiotic-associated diarrhea and supporting microbiome restoration. These strains have shown an ability to adhere to the intestinal lining, resist stomach acid and bile salts, and reduce inflammation. Their protective action includes reinforcing gut barrier integrity, producing antimicrobial compounds that target harmful bacteria, and encouraging the growth of native commensal flora.
Administering the best probiotic for antibiotics during treatment can therefore mitigate side effects and improve outcomes. Beyond symptom relief, this approach may also reduce the risk of long-term gut dysbiosis—a condition increasingly linked to chronic health disorders. Moreover, when considering why use probiotics instead of antibiotics for certain non-life-threatening ailments, it’s often because probiotics help promote long-term immune modulation and gut ecosystem balance without contributing to antibiotic resistance. The synergistic rather than antagonistic relationship between probiotics and antibiotics is now well-established, provided that use is well-informed and appropriately managed.
Best Probiotic for Antibiotics: Strains That Truly Matter
Not all probiotics are created equal. While the marketplace is flooded with a variety of options claiming digestive support, only certain strains have demonstrated consistent effectiveness in clinical settings when paired with antibiotic therapy. Choosing the best probiotic for antibiotics means identifying formulations that include robust, scientifically validated strains with the ability to survive gastrointestinal transit and recolonize the gut effectively.
Lactobacillus rhamnosus GG stands out due to its documented ability to reduce antibiotic-associated diarrhea and its role in strengthening gut barrier function. Its adhesion properties allow it to colonize the intestines temporarily and provide immediate benefits during and after antibiotic treatment. Another standout strain is Saccharomyces boulardii, a beneficial yeast that is naturally resistant to antibiotics. Its unique status as a yeast-based probiotic allows it to function alongside antibacterial medications without being destroyed, making it an ideal companion during therapy. It has been shown to reduce the incidence of Clostridium difficile infections, particularly in hospital settings where such risks are elevated.
Bifidobacterium lactis and Lactobacillus acidophilus are additional strains frequently included in combination probiotics due to their synergy with other beneficial microbes. These strains help produce short-chain fatty acids like butyrate, which serve as energy sources for colon cells and reduce inflammation. When selecting a probiotic, it’s essential to consider both the CFU count (colony-forming units) and the diversity of strains included. A multi-strain formulation may provide more comprehensive support, as different strains colonize different parts of the gut and offer varied functional benefits.
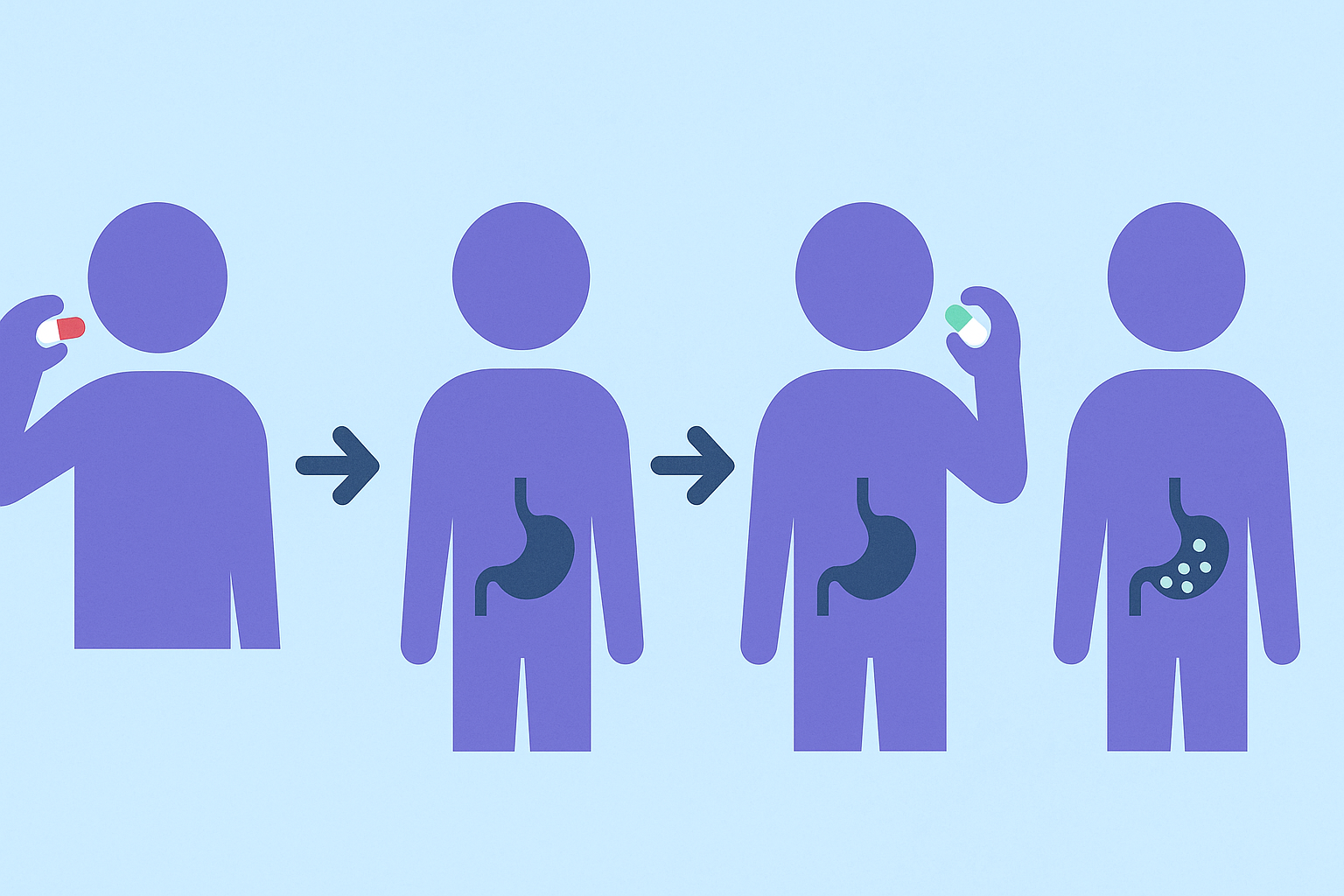
Probiotics for Taking Antibiotics: Timing, Dosage, and Delivery
Effective use of probiotics with antibiotics hinges on timing. To maximize survival and colonization, it is generally recommended to take probiotics two to three hours after the antibiotic dose. This delay helps reduce the likelihood that the probiotic organisms will be neutralized by the medication. Continuing probiotic use for at least one to two weeks after completing the antibiotic course further enhances gut flora recovery.
Dosage also matters. While research is ongoing, many experts recommend choosing probiotics that contain at least 10 billion CFUs per serving during antibiotic use. Higher doses may be appropriate in cases of significant gut distress or for individuals with a history of antibiotic-associated complications. However, more is not always better—quality, viability, and strain specificity should guide the decision more than sheer quantity.
The method of delivery is another critical consideration. Capsules with enteric coating help ensure the probiotics survive the acidic environment of the stomach and reach the intestines intact. Some formulations use microencapsulation technologies to further enhance stability. For those unable to swallow capsules, powders and chewables offer alternatives, though they must be formulated to preserve microbial viability. Ultimately, the best probiotic for antibiotics is one that delivers its promised strains alive and in sufficient quantities to exert their health-promoting effects.
Why Use Probiotics Instead of Antibiotics in Some Cases?
While antibiotics remain essential for treating bacterial infections, they are not always the best choice for every scenario. Overprescription and inappropriate use have contributed to antibiotic resistance—a growing public health threat. In cases of viral infections or mild gastrointestinal complaints not caused by pathogenic bacteria, probiotics can serve as a more appropriate first line of defense.
Probiotics help restore and maintain a balanced gut microbiome, which in turn supports the immune system and reduces inflammation. For example, individuals suffering from irritable bowel syndrome (IBS) or inflammatory bowel diseases (IBD) may benefit more from targeted probiotic therapies than from recurring antibiotic use, which could exacerbate dysbiosis. Moreover, probiotics can also help prevent recurring infections by fortifying the body’s natural defenses, reducing the need for repeated antibiotic interventions.
This approach reflects a growing trend in functional and integrative medicine—treating the root cause rather than just the symptoms. It underscores a paradigm shift from reactive to proactive health management. In this context, using probiotics with antibiotics becomes part of a broader strategy of microbial stewardship, aiming to harness the body’s innate healing capabilities while minimizing collateral damage to its beneficial flora.
Choosing the Right Probiotic Formulation: Key Considerations
Selecting the best probiotic for antibiotics involves more than just reading the label. Consumers must consider strain specificity, manufacturing quality, third-party testing, and the presence of any additional ingredients that may influence efficacy. A well-formulated probiotic will list not only the species but also the specific strains included—such as Lactobacillus rhamnosus GG rather than just Lactobacillus rhamnosus—since clinical studies are typically strain-specific.
Another crucial factor is shelf stability. Some probiotics require refrigeration to maintain potency, while others are shelf-stable due to advanced encapsulation technologies. It’s important to store these products according to manufacturer guidelines to preserve their effectiveness. Additionally, check for certifications such as NSF, USP, or other third-party verifications that assure manufacturing quality and microbial viability.
Added ingredients can either enhance or detract from the product’s effectiveness. Prebiotics like inulin or fructooligosaccharides can support the growth of probiotics, while fillers, artificial sweeteners, and allergens may undermine their benefits for sensitive individuals. Transparent labeling and clear usage instructions indicate a brand’s commitment to consumer education and product integrity.
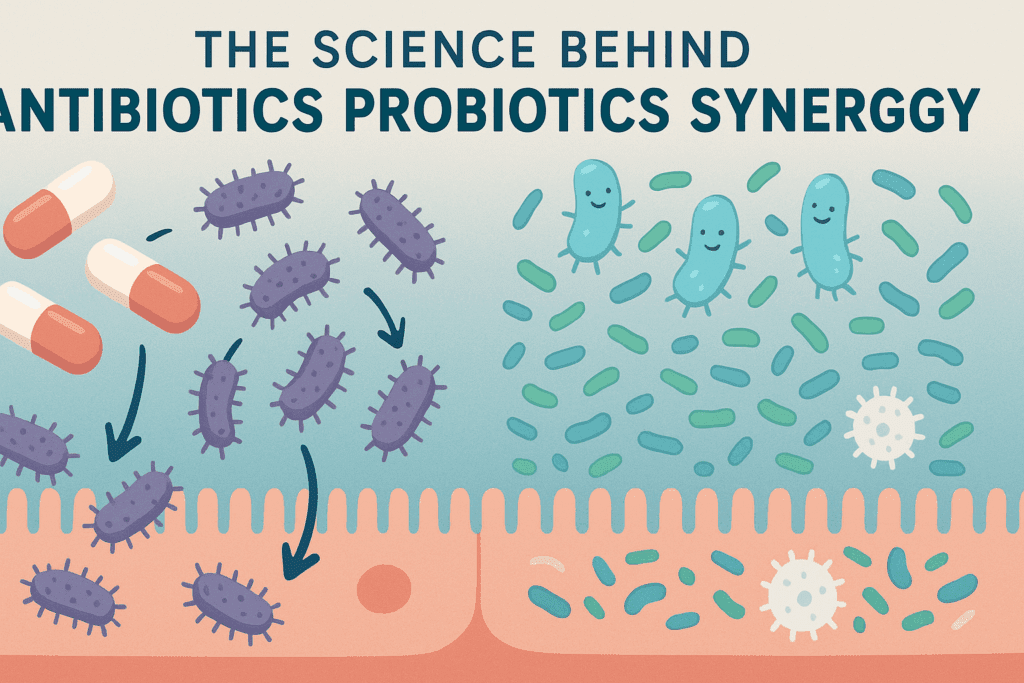
The Science Behind Antibiotics Probiotics Synergy
One of the most compelling reasons for integrating probiotics during antibiotic therapy is the robust body of scientific literature supporting this practice. Clinical trials have consistently shown that probiotics can reduce the risk of antibiotic-associated diarrhea by up to 42%. They also demonstrate effectiveness in preventing Clostridioides difficile infections, particularly in vulnerable populations such as the elderly and those with compromised immunity.
The mechanisms behind this protective effect are multifaceted. Probiotics help compete with pathogenic bacteria for nutrients and adhesion sites on the gut lining, thereby reducing colonization by harmful microbes. They also stimulate the production of mucus, defensins, and immunoglobulins, all of which strengthen gut barrier function. Furthermore, probiotics influence dendritic cell activity and cytokine production, fostering an anti-inflammatory environment conducive to healing.
Emerging research using next-generation sequencing and metabolomic analysis has revealed that probiotic supplementation during antibiotic treatment can accelerate the reestablishment of microbial diversity. It also helps maintain the presence of keystone species critical to short-chain fatty acid production and gut health. The convergence of these findings provides a compelling rationale for adopting a dual strategy of using antibiotics for infection control and probiotics for microbial recovery.
Practical Tips for Integrating Probiotics During Antibiotic Therapy
Integrating probiotics into an antibiotic regimen doesn’t have to be complicated, but it does require a thoughtful approach. Start by identifying the timing of your antibiotic doses and insert the probiotic intake two to three hours later. Maintain consistency throughout the course of antibiotics and extend probiotic use for at least 7 to 14 days afterward to support full microbiome restoration.
Choose a probiotic with well-researched strains like Saccharomyces boulardii or Lactobacillus rhamnosus GG, and ensure that the product has been stored properly to preserve viability. For children or individuals who have difficulty swallowing pills, opt for flavored powders or chewables, keeping in mind that not all delivery forms are equally effective.
Support your probiotic regimen with a gut-friendly diet rich in prebiotic fibers found in foods like bananas, garlic, onions, asparagus, and oats. These fibers serve as fuel for beneficial bacteria, encouraging their growth and activity. Also, minimize processed foods and excessive sugar intake, which can foster the growth of harmful microbes and undermine the effects of probiotic supplementation. Together, these strategies create a supportive environment in which probiotics can thrive and restore balance effectively.
Frequently Asked Questions (FAQ)
1. Can taking probiotics reduce the likelihood of antibiotic resistance?
Yes, certain probiotics may help minimize the development of antibiotic resistance by reducing the frequency and severity of infections that require antibiotic treatment in the first place. For example, probiotics such as Lactobacillus plantarum and Bifidobacterium breve have shown immunomodulatory effects that enhance mucosal defenses, potentially preventing recurrent bacterial infections. When infections are prevented or resolved more efficiently, there is less reliance on antibiotics, which in turn reduces the pressure that drives resistance. Additionally, some strains may neutralize antibiotic-resistant genes in the gut microbiome, although this area is still being actively researched. Integrating probiotics for taking antibiotics into a broader preventative care routine is a promising strategy to protect public health in an era of rising antimicrobial resistance.
2. What are some emerging probiotic delivery systems that enhance absorption during antibiotic treatment?
Innovations in probiotic delivery systems are addressing challenges such as gastric acid degradation and inconsistent viability. Microencapsulation is one such advancement where probiotic strains are coated in a protective polymer to improve survivability through the stomach and release them in the intestines. Another breakthrough is time-release capsules that deploy probiotics at intervals aligned with digestive transit, minimizing overlap with antibiotic action. Researchers are also exploring symbiotic formulations that combine prebiotics and postbiotics to create a hospitable environment for colonization. These delivery technologies are critical in determining the best probiotic for antibiotics, as survival through the digestive tract is essential for any meaningful therapeutic effect.
3. Are there specific risks when combining probiotics and antibiotics in immunocompromised individuals?
While most people benefit from using probiotics with antibiotics, those with severely weakened immune systems—such as patients undergoing chemotherapy or organ transplant recipients—should proceed with caution. In rare cases, live probiotics can translocate into the bloodstream and cause infections, particularly if gut integrity is compromised. For such individuals, heat-killed or non-viable probiotic products are being explored as alternatives that retain some immunomodulatory benefits without the risks associated with live cultures. These patients should only consider probiotics under medical supervision and choose strains with extensive clinical data on safety, especially when evaluating probiotics for taking antibiotics. The decision must be individualized based on immune status, underlying conditions, and the type of antibiotic used.
4. How can dietary choices enhance the effects of probiotics during antibiotic therapy?
Diet can significantly influence how well probiotics function during and after a course of antibiotics. Foods rich in fermentable fibers, such as garlic, leeks, and Jerusalem artichokes, provide prebiotics that fuel beneficial bacteria. Consuming fermented foods like kimchi, kefir, and miso can introduce a variety of microbial species and support recolonization efforts. On the other hand, diets high in sugar, alcohol, or processed ingredients can hinder probiotic effectiveness by encouraging the growth of opportunistic pathogens. Individuals looking for the best probiotic for antibiotics should also consider how their food choices either reinforce or sabotage their microbiome. A diet that supports microbial diversity creates an environment where supplemental probiotics can thrive and exert their beneficial effects more efficiently.
5. What does current research say about the long-term effects of taking probiotics after antibiotics?
Long-term studies on probiotics following antibiotics are uncovering some surprising insights. While short-term supplementation clearly benefits gut recovery, research suggests that continuing probiotics for 30 to 60 days post-antibiotics may lead to even greater microbial diversity and resilience. Some findings indicate that probiotic supplementation may help reestablish species that antibiotics suppress for weeks or even months. Moreover, the sustained intake of specific probiotics could influence metabolic health, such as improved glucose regulation and reduced systemic inflammation. This aligns with broader interest in probiotics for taking antibiotics as part of an extended recovery plan rather than just an acute phase intervention. Ultimately, ongoing use may provide a bridge to reestablishing gut equilibrium and preventing dysbiosis-related health conditions in the future.
6. What should you avoid when choosing a probiotic during antibiotic treatment?
Not all probiotics are equally beneficial, and some may be ineffective or even counterproductive during antibiotic therapy. Avoid products that do not specify the strain, as benefits are often strain-dependent and generic labeling can be misleading. Steer clear of probiotics with low CFU counts—less than 5 billion CFU per dose is generally insufficient for therapeutic impact during antibiotic use. Products with added sugars, artificial sweeteners, or synthetic fillers may reduce gut barrier integrity and counteract the benefits of good strains. Most importantly, don’t rely on yogurts or general “gut health” supplements unless they’ve been clinically studied for use alongside antibiotics. The best probiotic for antibiotics will always be supported by evidence and clearly labeled for strain identity, potency, and viability at the time of consumption.
7. How do individual genetics influence your response to probiotics and antibiotics?
Genetic variability can influence how one’s body processes both antibiotics and probiotics, including how the microbiome reacts to interventions. Some individuals have gene variants that affect bile acid metabolism or mucosal immunity, which in turn modulate how well probiotics can colonize the gut. Differences in enzyme production and immune receptor expression also determine how the body tolerates or rejects certain microbial strains. Personalized probiotics, guided by microbiome sequencing or pharmacogenomics, are emerging as a frontier in precision medicine. In time, genetic profiling may inform whether using probiotics with antibiotics is likely to be beneficial for a specific person, offering a tailored approach to microbial support based on one’s unique biology.
8. How do environmental and lifestyle factors impact probiotic effectiveness during antibiotic use?
Environmental toxins, stress levels, physical activity, and sleep patterns all have a cumulative effect on the gut microbiome and how it responds to probiotic interventions. Chronic stress, for example, increases cortisol levels that disrupt gut barrier function and reduce microbial diversity, making it harder for probiotics to take hold. Similarly, sedentary lifestyles and irregular sleep cycles can slow digestion and reduce gut motility, impairing probiotic transit and colonization. Those living in highly polluted environments or using personal care products with antibacterial agents may also experience a weakened microbiome. Addressing these lifestyle factors is essential when integrating antibiotics probiotics strategies, ensuring that the benefits of supplementation are not undermined by preventable external stressors.
9. Why use probiotics instead of antibiotics for recurring minor infections?
When dealing with minor, non-severe infections—especially those that are viral or inflammatory in nature—probiotics can offer a more balanced, supportive approach. For example, conditions like recurrent urinary tract infections or sinus inflammation may benefit from targeted probiotics that reduce pathogenic adhesion and boost mucosal immunity. Overuse of antibiotics in such cases often exacerbates the problem by disrupting native flora and fostering resistant strains. Opting for probiotics instead of antibiotics, when clinically appropriate, helps preserve the microbiome and may prevent escalation to more serious infections. The growing body of evidence supporting this approach is shifting how clinicians think about long-term care and prevention, emphasizing the protective power of probiotics for taking antibiotics only when absolutely necessary.
10. What future innovations might redefine the best probiotic for antibiotics?
The future of probiotics is moving toward precision, personalization, and advanced delivery. Innovations include genetically engineered probiotics that produce specific enzymes or signaling molecules to repair gut tissue or modulate immune responses. Other breakthroughs involve artificial intelligence platforms that recommend custom probiotic blends based on microbiome sequencing. Encapsulation technologies using biodegradable nanomaterials are in development to enhance targeted delivery in the colon rather than the stomach or upper intestine. Additionally, postbiotics—metabolites produced by probiotics—are gaining traction as potentially more stable and equally beneficial alternatives. These emerging trends suggest that the definition of the best probiotic for antibiotics is poised to evolve rapidly, incorporating next-generation science to provide deeper, more effective gut recovery solutions.
Conclusion: A Proactive Path to Gut Health with the Best Probiotic for Antibiotics
In the journey toward recovery and wellness, antibiotics and probiotics are not adversaries but allies—each with distinct yet complementary roles. While antibiotics target and eliminate harmful pathogens, they also disrupt the delicate microbial ecosystem within the gut. This collateral damage can lead to uncomfortable symptoms and longer-term health risks if not addressed. Integrating the best probiotic for antibiotics into your recovery plan offers a scientifically grounded and clinically validated strategy to protect and restore gut health. It ensures that while you’re fighting infection, you’re also laying the groundwork for a resilient, thriving microbiome.
Choosing the right probiotic requires attention to detail, scientific literacy, and an understanding of your individual health needs. The synergy between antibiotics probiotics is now well recognized in both clinical practice and health-conscious communities. Whether you’re seeking to prevent digestive upset, support immune function, or reduce the risk of secondary infections, the thoughtful use of probiotics provides an empowering, evidence-based tool for optimizing outcomes. By appreciating the nuances of timing, strain selection, and dietary support, you take an active role in shaping your recovery—not just from illness, but toward lasting vitality.
Further Reading:
The Essential Guide to Taking Probiotics with Antibiotics: What You Need to Know
How Much Probiotic to Take with Antibiotics: A Comprehensive Guide to Gut Health